Course Chemical Bonds: Covalent Bonding and Shapes of Molecules (20)
sp Hybrid Orbitals—Bond Angles of Approximately 180°
In sp hybridization, only one p orbital participates in the hybridization with the s orbital.
If we looked at sp hybridization of carbon atom, carbon’s 2s orbital hybridizes with one of the 2p orbitals, resulting in the formation of two sp hybridized orbitals, while the other two 2p orbitals remain unhybridized.
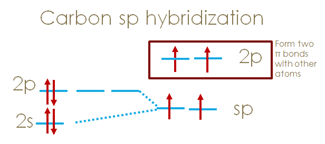
The hybridization of one s and one p orbital yields two sp orbitals with 50% s character and 50% p character.

The angle between the two hybridized orbitals is 180 degrees, indicating that both sp orbitals lie on the same plane, while the two unhybridized 2p orbitals are perpendicular to the sp hybridized orbitals.

Notice that, the unhybridized 2p orbitals are the ones form pi bonds, that is why we expect two pi bonds.
Acetylene molecule
Let’s examine the acetylene molecule. Each carbon atom in acetylene possesses two sp hybridized orbitals and two unhybridized 2p orbitals, totaling four sp orbitals and four 2p orbitals for the two carbon atoms.

Acetylene also contains two hydrogen atoms with two 1s orbitals.
The molecule’s bonds consist of:
Two sigma C-H bonds formed by overlapping the two sp orbitals of each carbon with the hydrogen 1s orbitals.
One sigma C-C bond formed by overlapping the two sp carbon orbitals of each carbon.
Two C-C pi bonds formed by overlapping the four 2p orbitals.

In total, acetylene comprises five bonds: two sigma carbon-hydrogen bonds, one sigma carbon-carbon bond, and two carbon-carbon pi bonds.
Read about:
sp3 Hybridization: Covalent Bonds (18)
sp2 Hybridization: Covalent Bonds Course (19)
Full Course Link: Covalent Bonding and Shape of Molecules
Subscribe to get updated when we upload a new lecture