Principles of Polymer Chemistry: Monomers, Polymers, And Macromolecules An Overview (Lect 1)
Polymers are very interesting materials. Although polymers are one of the building blocks of life on Earth, we only recently understood their nature.

The origin of the word “polymer” comes from the Greek word “polymeros,” which means “many parts.” Polymers are formed by linking small monomers, from the Greek word “monomeros,” meaning “one single part,” together to form long-chain polymers.
The first synthetic polymer was introduced by Leo Baekeland in 1907.

Leo Baekeland 1907
He was able to synthesize a thermosetting phenol-formaldehyde resin called Bakelite.

Bakelite products: Thermosetting phenol–formaldehyde resin “Bakelite”
Although there were significant advances in polymer plastic synthesis, the molecular nature of polymers was not understood. It was believed that polymers were formed according to association theory, which suggested that polymers were created by the association or aggregation of monomers.

Association or aggregation theory
It was not until Hermann Staudinger proposed in 1922 that polymers consist of long chains of atoms held together by covalent bonds.

Hermann Staudinger 1922
His work was not accepted until 1953, when he was awarded the Nobel Prize.

Polymers consist of long chains of atoms held together by covalent bonds
What is the definition of a polymer?
A polymer is a molecular compound distinguished by a high molecular mass, ranging between thousands to millions of grams per mole, and is made up of many repeating units.

Example
Polyethylene [–CH2CH2–]n is a polymer made from repeating units of ethylene (CH2=CH2) monomers. Polymers are also called macromolecules due to their large size and high molecular weight.

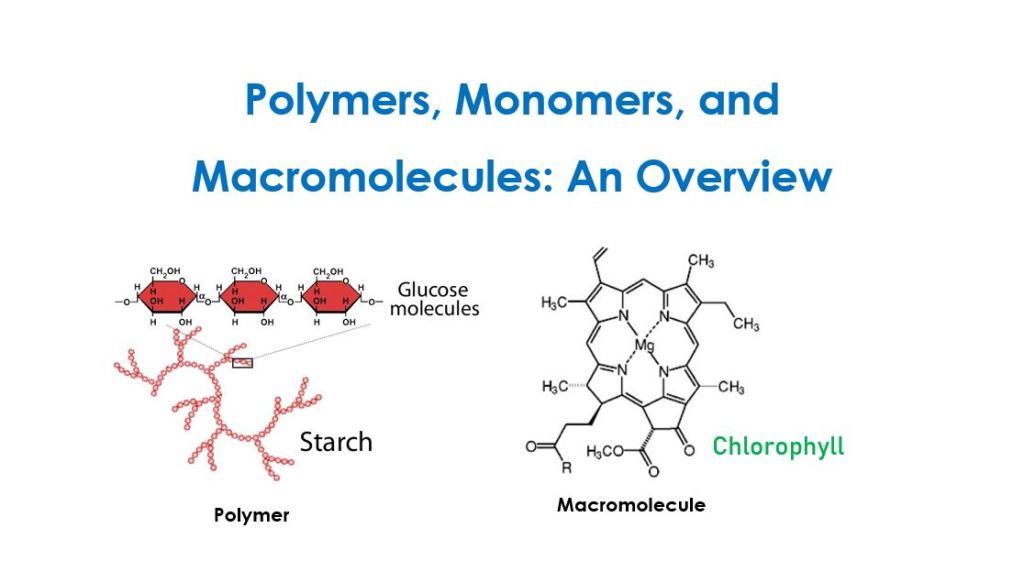
All polymers are considered macromolecules, but not all macromolecules are considered polymers. So what is the difference?
Polymers = Macromolecules
Macromolecules ≠ Polymers
All polymers are considered macromolecules due to their large size and high molecular weight. Additionally, polymers are made of small monomers joined together. For example, starch is a polysaccharide made by linking glucose monomers together.
In contrast, macromolecules do not have to be polymers. For example, chlorophyll is a macromolecule with a high molecular weight, yet it is not considered a polymer. The structure of chlorophyll does not contain monomers; instead, it is a single large compound. In other words, it is not made by joining monomers together.
In the next lecture let discuss in details the classification of polymers.
Full Course Link:
Principles of Polymer Chemistry: Master the Basics of Polymer Chemistry
Subscribe to get updated when we upload a new lecture