Hydrocarbons are carbon-containing compounds. They are organic compounds that contain C-C and C-H bonds.
Hydrocarbons classification is based on the degree of bond saturation.
Classification of hydrocarbons:
Alkanes are saturated hydrocarbons containing C-C or C-H sigma bonds.

They have the general formula CnH2n+2. This means for each carbon, there are four hydrogens. For example, a compound that contains three carbons will contain a number of hydrogen equal to:
2n+2 = (2 X C) + 2 = (2 X 3) + 2 = 8 H
The name of alkanes consists of the suffix “ane” added to Greek numbers (except for the first four members):
Methane is derived from the Greek word for wine (methu).
Ethane is derived from the Greek word (aether)
Propane is derived from the word (protos)
Butane is derived from the word (butyrum)
The rest of the alkanes consist of the suffix “ane” added to Greek numbers.
| Name | MolecularFormula | CondensedStructural | Formula NameMolecular | FormulaCondensed | StructuralFormula |
| Methane | CH4 | CH4 | Undecane | C11H24 | CH3(CH2)9CH3 |
| Ethane | C2H6 | CH3CH3 | Dodecane | C12H26 | CH3(CH2)10CH3 |
| Propane | C3H8 | CH3CH2CH3 | Tridecane | C13H28 | CH3(CH2)11CH3 |
| Butane | C4H10 | CH3(CH2)2CH3 | Tetradecane | C14H30 | CH3(CH2)12CH3 |
| Pentane | C5H12 | CH3(CH2)3CH3 | Pentadecane | C15H32 | CH3(CH2)13CH3 |
| Hexane | C6H14 | CH3(CH2)4CH3 | Hexadecane | C16H34 | CH3(CH2)14CH3 |
| Heptane | C7H16 | CH3(CH2)5CH3 | Heptadecane | C17H36 | CH3(CH2)15CH3 |
| Octane | C8H18 | CH3(CH2)6CH3 | Octadecane | C18H38 | CH3(CH2)16CH3 |
| Nonane | C9H20 | CH3(CH2)7CH3 | Nonadecane | C19H40 | CH3(CH2)17CH3 |
| Decane | C10H22 | CH3(CH2)8CH3 | Eicosane | C20H42 | CH3(CH2)18CH3 |
Methane
Methane is the first member of the alkanes family; it contains one single carbon and has the formula (CH4).
Methane forms a tetrahedral structure with an H-C-H bond angle of 109.5o. We can draw methane using a line-angle formula as:

We also can use the ball-and-stick model to show the tetrahedral structure and the H-C-H angle:

Although the rest of the alkanes have multiple bonds and more complex structures, they all have their carbons surrounded by four bonds at 109o angles.
For example, ethane has two carbons, each surrounded by four bonds, forming a tetrahedral structure around each carbon.
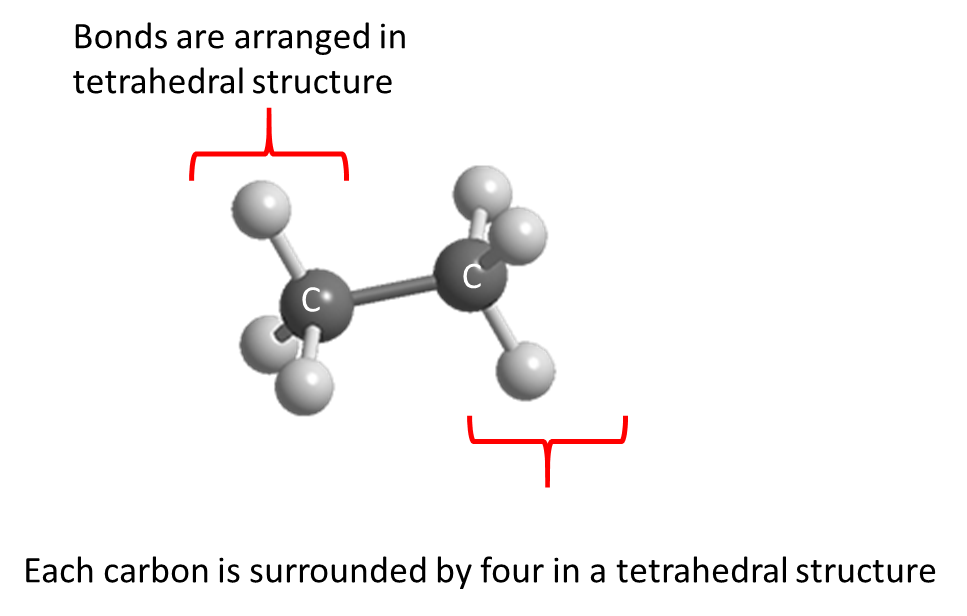
Notice the sum of angles around the carbon is 438o, not 360o. Although you may think four bonds mean 360o/4 = 90o each, the actual angles are 109o each. This happened because bonds arranged in 3D space can move out of the plane. Therefore, angles can be larger than 90o.
You have the opportunity to enroll in a comprehensive course covering Hydrocarbons Chemistry.